Basic Concepts in Thermodynamics
Basic Concepts in Thermodynamics: Overview
This Topic covers sub-topics such as Chemical Energy Transformations, Thermodynamic Equilibrium State and, Common Terms in Thermodynamics
Important Questions on Basic Concepts in Thermodynamics
Which of the following are not state functions?
During isothermal expansion of an ideal gas, its
For a cyclic process, which of the following is not true?
At constant temperature, for a given mass of an ideal gas
ice at is mixed with steam at . The final temperature of the mixture is.
The total number of intensive properties from the following is _____.
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
What happens when methane undergoes combustion in systems A and B respectively?
Number of intensive properties are:
, Molarity, Gibbs free energy, Molar mass, Mole, Molar heat capacity?
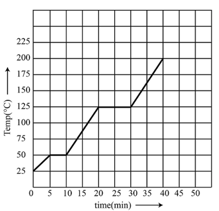
The graph shown in the figure represent change in the temperature of of a substance as it absorbs heat at a constant rate of . The latent heat of vaporization of the substance is
of ice at is mixed with of water at in a calorimeter. If the specific heat of ice and water are respectively and the latent heat of ice is , the temperature of the mixture in thermal equilibrium in Kelvin is
Round off the answer up to two decimal places.
Is work done intensive or extensive property?
The container that suits the occurence of an isothermal process should made of
Observe the following properties:
Volume, enthalpy, density, temperature, heat capacity, pressure, internal energy.
The number of extensive properties in the above list is
Establish the adiabatic equation constant.
What is a thermodynamic system?
Out of three quantities in thermodynamics first law namely and , which one depends on path and which one does not?
Define the boundary of a system.
Define surroundings in thermodynamics.
Three separate reversible processes are carried out with mole of an ideal gas initially at STP. In these processes, the pressure () is measured while increasing any one of the quantities volume (), temperature () and number of moles , keeping the other two constant. Plots of versus are found to be linear, where or or . For the processes and , the numerical values of the slopes and of the versus plots are found to follow the order , respectively. The correct statement(s) about these processes is/are
Consider a cyclic process for a system. Among the following, the combination of steps that can never lead to this cyclic process is
